Cell
The cell is a device which converts chemical energy in to electrical energy .cell produce energy is DC .If two different plates or electrode are immersed in electrolyte solution like dilute acid, an e.m.f. is set up in the two electrodes. If the two plates are connected by a piece of wire, current starts flowing from one electrode to another side .
main parts of cell is :
(a) Electrode
(b) Electrolyte
(C) container
There are two types of cell
1) primary cell
2) secondary cell
1.primery cell : Primary cells are those cells that are not Rechargeable. , the chemical reaction that occurs during discharge is not easily reversed. When the chemicals used in the reactions are all converted, the cell is fully discharged. It must then be replaced by a new cell. Included in the primary cell category are the following types.
--Voltaic cell
--Carbon-zinc (Leclan he cell) (Dry cell)
--Alkaline
--Mercury
--Silver oxide
• voltaic cell:
A voltaic cell uses copper and zinc as the two electrodes and sulphuric acid as the electrolyte. When placed together a chemical reaction occurs be- tween the electrodes and the sulphuric acid. This reaction produces a negative charge on the zinc (surplus of electrons) and a positive charge on the copper (deficiency of electrons). If an external circuit is connected across the two electrodes, electrons will flow from the negative zinc electrode to the positive copper electrode. (Fig 1) The electric current will flow as long as the chemical action continues. In this type of cell the zinc electrode is eventually consumed as part of the chemical reaction.
The voltaic cell is also known as a wet cell because it uses a liquid solution for the electrolyte
Leclanche cell (carbon zinc ) :
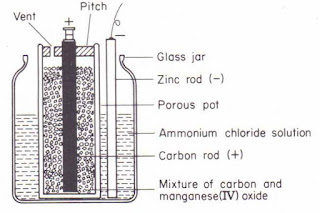
leclanche cell consist The container of this cell is a glass jar. The jar contains a strong solution of ammonium chloride (NH⁴,CI). This solution is an alkali and acts as the electrolyte. A porous pot is placed at the centre of the glass jar. This porous pot has in it a carbon rod surrounded by a mixture of manganese dioxide (MnO₂) and powdered carbon. The carbon rod forms the positive electrode of the cell and MnO, acts as the de-polarizer. A zinc rod is dipped in the solution in the jar and acts as the negative electrode.
Dry cell : The danger of spilling the liquid electrolyte from a Leclanche type of cell led to the invention of another class of cells called dry cells.
The most common and least expensive type of a dry cell battery is the carbon-zinc type. This cell consists of a zinc container which acts as the negative electrode. In the centre is a carbon rod which is the positive elec-trode. The electrolyte takes the form of a moist paste made up of a solution containing ammonium chloride. As with all primary cells, one of the electrodes becomes decomposed as part of the chemical reaction. In this cell the negative zinc container electrode is the one that is used up. As a result, cells left in equipment for long periods of time can rupture, spilling the electrolyte .
Alkaline cells: Alkaline cells is a cell that use a zinc container for the negative electrode and a cylinder of manganese di-oxide for the positive electrode. The electrolyte is made up of a solution of potassium hydroxide or an alkaline solution
Alkaline cells are produced in the same standard sizes as carbon-zinc cells but are more expensive. They have the advantage of being able to supply large currents for a longer period of time. For example, a standard 'D' type 1.5 Valkaline cell has a capacity of about 3.5 A.h compared with about 2 A.h for the carbon-zinc type. A second advantage is that the alkaline cell has a shelf life of about two and a half years as compared to about 6 to 12 months for the carbon-zinc type.
Mercury cells: Mercury cells are most often used in digital watches, calculators, hearing aids and other mini- ature electronic equipment. They are usually smaller and are shaped differently from the carbon-zinc type
Silver oxide cell : Silver oxide cells are much like
mercury cells. However, they provide a higher voltage (1.5 V) and they are made for light loads. The loads can be continuous, such as those encountered in hearing aids and electronic watches. Like the mercury cell, the silver oxide cell has good energy-to-weight and energy-to-volume ratios, poor low-termperture response, and flat output voltage characteristics. The structures of the mercuric and silver oxide cells are very similar. The main difference is that the positive electrode of the silver cell is silver oxide.
Lithium cells: The lithium cell is another type of primary cell. It is available in a variety of sizes and configurations. Depending on the chemicals used with lithium, the cell voltage is between 2.5 and 3.6 V. Note that this voltage is considerably higher than in other primary cells. Two of the advantages of lithium cells over other primary cells are:
longer shelf life - up to 10 years higher energy-to-weight ratios up to 350 Wh/Kg.
Lithium cells operate at temperatures ranging from -50 to +75°C. They have a very constant output voltage during discharge.
SECONDARY CELL :
A cell can be recharged by sending electric current in the reverse direction to that of discharge mode is known as a secondary cell and secondary cell is also called a storage cell since after it is charged it stores the energy until it is used up .
In a secondary cell the charging and discharging proc- esses are taking place according to Faradays Laws of Electrolysis.
Terms and definitions :
Electrolyte: Electrolyte is a solution or paste capable of
conducting an electric current because of its dissociation into positive and negative ions.
Electrodes: The conductor or terminal, usually metallic,
through which an electric current enters or leaves is
called an electrode.
Anode: One terminal of a device (such as battery or electron tube) that loses electrons in the external circuit during normal operation is called the anode. In a battery, the negative electrode is the anode and the positive electrode is the cathode.
Electrolysis: The process of decomposing liquids by the passage of electric current through the liquid is called electrolysis.
Electrochemical equivalent: The mass of a substance liberated from an electrolyte by one coulomb of electric- ity is termed as electrochemical equivalent (ece) of that substance.
The ece of silver is 1.1182 milligram/coulomb.
Coulomb: The unit of electric charge is a coulomb. The quantity of electricity is otherwise called charge (Q) and its unit is coulomb (C).
The coulomb is the product of current in ampere and time in seconds.
THERE ARE TWO TYPES OF SECONDARY CELL :
- lead acid cell
- alkaline cell (iron nickel cell )
1) lead acid cell :
Following parts of lead acid cell:
1. Container
2. Plates
3. Separator
4. Post terminals
Container:
The container is made on hard rubber, glass or celluloid to accommodate the active plates, separa- tors and the electrolyte. The plates rest on ribs provided at the bottom of the container, and the space between ribs is known as sediment chamber.
Plates:
Positive plates are of two types.
Plante plate or formed plates
Faure plate
Plante plates: These are prepared by the process of
repeated charging and discharging. They are made of pure lead at the begining which changes to lead peroxide after charge.
Faure plate: Pasted or Faure plates are made of rectangular lead grid into which the active material i.e. lead peroxide (Pb O₂) is filled in the form of a paste
Separators: These are made of thin sheets of chemi- cally treated porous wood or rubber. They are used to avoid short in between the positive and negative plates.
Post terminal: A small pole extended upward from each group of welded plates from the plate connecter forms the post terminal.
Working principle: The secondary cell has no significant electrochemical energy at the start. The energy must first be charged into the secondary cell. Then the cell retains the stored energy until it is used up. That is, both cell electrodes are basically lead sulphate (Pb SO.). When the cell is charged, due to chemical reaction taking place in it, the lead sulphate electrode change to soft or spongy lead (Pb) (negative plate) and the other electrode changes to lead peroxide (Pb O₂) (positive plate).
At the same time the electrolyte solution is strengthened and becomes mostly sulphuric acid (H,SO,)



.jpg)


.jpg)







Post a Comment